Much has happened in the world since our last news item in March. However, we have been able to continue our work, even experiments, and have made good progress over the spring and summer.
First, a manuscript describing measurements of Tris buffer solubilities in various salt solutions, and measurements of water activities of aqueous Tris, is in the final stages of review by J. Chem. and Eng. Data. These measurements, carried out by Pablo Lodeiro at GEOMAR and Flo Gregson at the Bristol Aerosol Research Centre, provide important information about the interactions of the buffer substance Tris with other salts. The results are bring used to improve the chemical speciation model of Tris buffer solutions (for the calculation of pH) that we are developing. In a separate study, water activities of Tris solutions at various temperatures have just been measured by our collaborators Tian Xiaomeng and Prof. Chak Chan at City University of Hong Kong. A programme of Harned Cell measurements to be carried out by WG 145 associate members Regina Easley (NIST) and Frank Bastkowski (PTB, Germany) is being finalised.
Second, at the Ocean Sciences meeting in San Diego in February we showed draft software for the calculation of the pH of Tris buffers in artificial seawater, and the K* of the carbonate system in standard seawater. The novel features of these models are: (i) the ability to vary the composition of the solution medium from seawater stoichiometry; (ii) the calculation of uncertainty contributions from all the individual elements of the model. The draft software is still available to try here. Since the meeting we have refined the uncertainty treatment, and a manuscript describing the methods and results is currently in preparation. Here is a result we’d like to show you: ranked uncertainty contributions (as percentages of the total calculated variance) to a model-calculated pH of Tris buffer in artificial seawater of salinity 35.
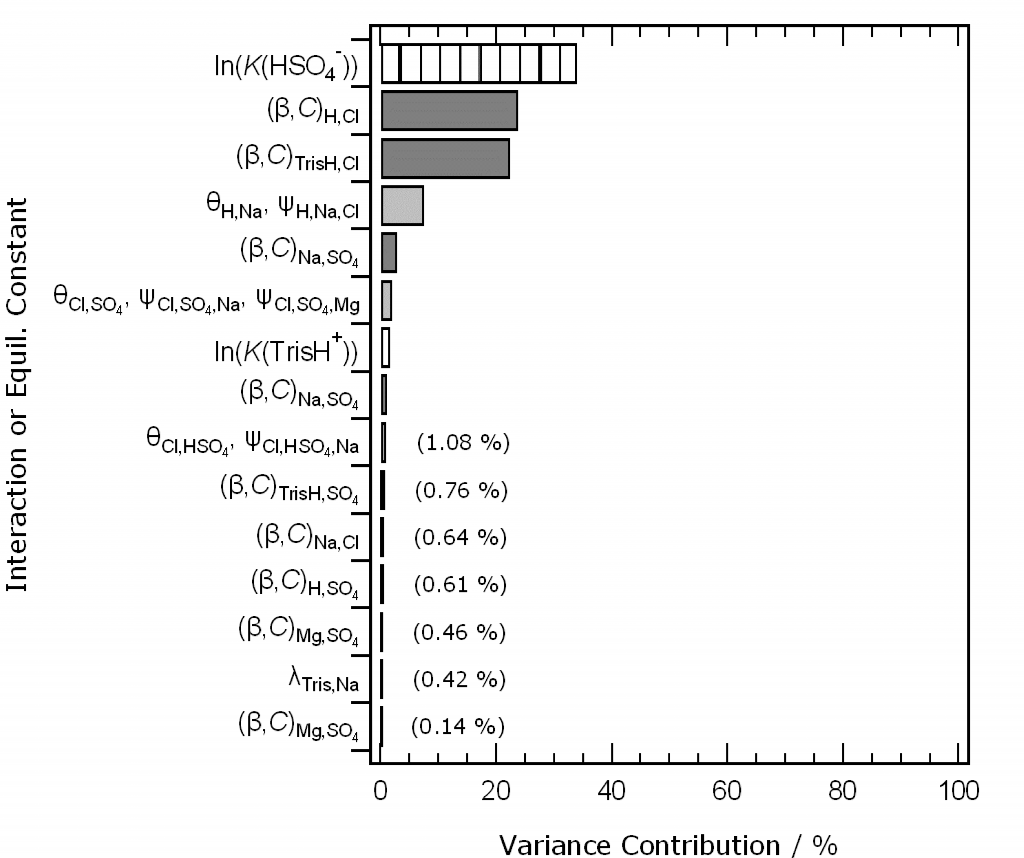
The key finding is that, of the very many interactions represented by parameters in the chemical speciation model, only a very few matter. This is extremely helpful for model development.
What about dissolved organic matter, and trace metals? Due to its polydisperse nature, the thermodynamics of natural organic matter (NOM) cannot be treated using the Pitzer approach that we are applying to standard seawater and Tris buffers. However, NOM is important as a trace metal complexant, and also as a contributor to titration alkalinity in lower salinity waters. Martha Gledhill and Pablo Lodeiro at GEOMAR have begun using the NICA-Donnan model, developed for freshwater NOM, to characterise the chemistry of marine NOM. Together with David Turner at the University of Gothenburg, they are assessing the use of a novel model code that combines the Pitzer and NICA-Donnan approaches. The first application to be studied is a new data set from the Amazon estuary.
At the recent SCOR Meeting the lifetime of WG 145 was extended for at least a further year in recognition of what we have achieved so far and of our current and planned activities.
