We have just submitted our first two modelling papers to Marine Chemistry (the abstracts are shown below).
The first manuscript concerns the model of artificial seawater, which is the core of any speciation model of seawater and related natural waters. It particularly focuses on acidified artificial seawater, because measurements of these solutions are essential to the definition of the ‘total’ pH scale for seawater, developed by WG 145 member Andrew Dickson.
The second manuscript describes the first speciation model of the Tris buffer solutions (equimolal Tris and TrisH+ in artificial seawater) used to define the total pH scale. We examine the assumptions inherent in the definition of the scale, the relationship of total pH to true total hydrogen ion concentration, and prospects for the extension of the scale to low salinities. There may be implications for the calculation of carbonate system equilibria from measured total pH.
Please get in touch with Simon Clegg (s.clegg@uea.ac.uk) or David Turner (david.turner@marine.gu.se) if you would like to know more.
Chemical Speciation Models Based Upon the Pitzer Activity Coefficient Equations, Including the Propagation of Uncertainties: Artificial Seawater from 0 to 45 oC
Matthew P. Humphreys, Jason F. Waters, David R. Turner, Andrew G. Dickson, and Simon L. Clegg
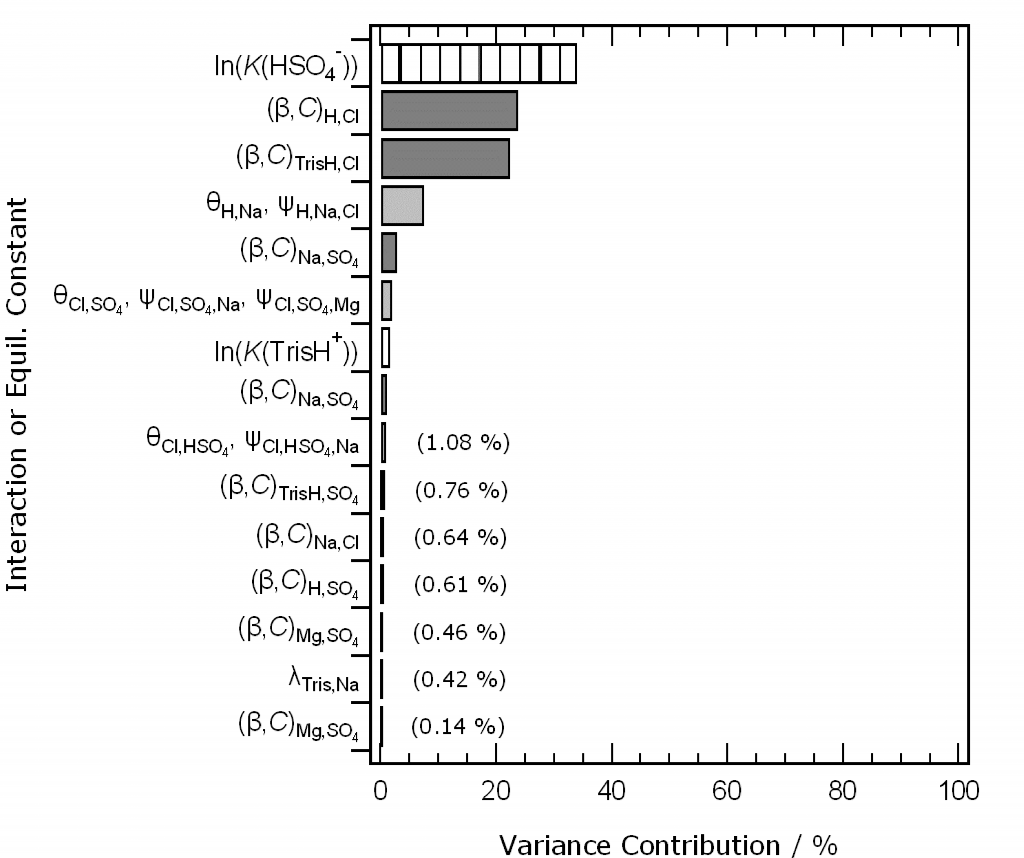
ABSTRACT: Accurate chemical speciation models of solutions containing the ions of seawater have applications in the calculation of carbonate system equilibria and trace metal speciation in natural waters, and the determination of pH. Existing models, based on the Pitzer formalism for the calculation of activity coefficients, do not yet agree with key experimental data (potentiometric determinations of H+ and Cl– activity products in acidified artificial seawaters) and, critically, do not include uncertainty estimates. This hampers both applications of the models, and their further development (for which the uncertainty contributions of individual ion interactions and equilibrium constants need to be known). We have therefore implemented the models of Waters and Millero (Mar. Chem. 149, 8-22, 2013) and Clegg and Whitfield (Geochim. et Cosmochim. Acta 59, 2403-2421, 1995) for artificial seawater, within a generalised treatment of uncertainties, as a first step towards a more complete model of standard seawater and pH buffers. This addition to the model enables both the total uncertainty of any model-calculated quantity (e.g., pH, speciation) to be estimated, and also the contributions of all interaction parameters and equilibrium constants. Both models have been fully documented (and some corrections made). Estimates of the variances and covariances of the interaction parameters were obtained by Monte Carlo simulation, with simplifying assumptions. The models were tested against measured electromotive forces (EMFs) of cells containing acidified artificial seawaters. The mean offsets (measured – calculated) at 25 oC for the model of Waters and Millero are: 0.046±0.11 mV (artificial seawater without sulphate, 0.280 mol kg-1 to 0.879 mol kg-1 ionic strength); and -0.199±0.070 mV (artificial seawater, salinities 5 to 45). Results are similar at other temperatures. These differences compare with an overall uncertainty in the measured EMFs of about 0.04 mV. Total uncertainties for calculated EMFs of the solutions were dominated by just a few contributions: mainly H+-Cl–, Na+-Cl–, and H+-Na+-Cl– ionic interactions, and the thermodynamic dissociation constant of HSO4–. This makes it likely that the accuracy of the models can readily be improved, and recommendations for further work are made. It is shown that calculated standard EMFs used in the definition of the marine ‘total’ pH scale can be accurately predicted with only slight modification to the original models, suggesting that they can contribute to the extension of the scale to lower salinities.
Chemical Speciation Models Based Upon the Pitzer Activity Coefficient Equations, Including the Propagation of Uncertainties. II. Tris Buffers in Artificial Seawater at 25 oC, and the Marine ‘Total’ pH Scale
Simon L. Clegg, Matthew P. Humphreys, Jason F. Waters, David R. Turner, and Andrew G. Dickson
ABSTRACT: The substance Tris (or THAM, 2-amino-2-hydroxymethyl-1,3-propanediol, CAS 77-86-1), and its protonated form TrisH+, is used in the preparation of pH buffer solutions for applications in seawater chemistry. The development of an acid-base chemical speciation model of buffer solutions containing Tris, TrisH+, and the major ions of seawater is desirable so that: (i) the effects of changes in the composition and concentration of the medium on pH can be calculated; (ii) pH on the free (a measure of [H+]) and total (a measure of ([H+] + [HSO4–])) scales can be interconverted; (iii) approximations inherent in the definition of the total pH scale can be quantified; (iv) electrode pairs such as H+/Cl– and H+/Na+ can more easily be calibrated for the measurement of pH. As a first step towards these goals we have extended the Pitzer-based speciation model of Waters and Millero (Mar. Chem. 149, 8-22, 2013) for artificial seawater extended to include Tris and TrisH+, at 25 oC. Estimates of the variances and covariances of the additional interaction parameters were obtained by Monte Carlo simulation (Humphreys et al., submitted to Mar. Chem.). This enables both the total uncertainty of any model-calculated quantity (e.g., pH, speciation) to be estimated, and also the individual contributions of all interaction parameters and equilibrium constants. This is important for model development, because it allows the key interactions to be identified. The model was used to quantify the difference between the operationally defined total pH scale and true -log10([H+] + [HSO4–]) in Tris buffer solutions at 25 oC, for the first time. The results suggest that the total pH scale can readily be extended to low salinities using the established approach for substituting TrisH+ for Na+ in the buffer solutions, especially if the speciation model is used to quantify the effect on pH of the substitution. The relationships between electromotive force (EMF), and pH on the total scale, with buffer concentration artificial seawater at constant salinity are shown to be linear over a reasonable range of buffer molality. The pH of Tris buffers containing ratios of TrisH+ to Tris that vary from unity can be very simply calculated (not requiring a model). Other technical aspects of the total pH scale, such as the extrapolation of pH to zero buffer (at constant salinity), are examined. The model was tested against measured EMFs of cells containing Tris buffer in artificial seawater at 25 oC, and the mean deviation (measured – calculated) was found to be 0.13±0.070 mV for salinities 20 to 40, using TrisH+-Cl– interaction parameters revised in this work. Total variances for calculated electromotive forces of the buffer solutions are dominated by contributions from just a few parameters, making it likely that the model can readily be improved with respect to accuracy. Recommendations for further work are made in order to extend the model to the 0 – 45 oC, and reduce errors to within or close to the experimental uncertainties of the data upon which the total pH scale is based.